Review: Technology and Techniques for Robotic-assisted Bronchoscopy
Jennifer D. Duke¹, Janani Reisenauer1,2*
1Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN, USA
2Division of Thoracic Surgery, Mayo Clinic, Rochester, MN, USA
Abstract
With recent advancements in robotic bronchoscopy for peripheral pulmonary nodule biopsy, there has been notable improvements in reach, stability, and precision. Since 2017, the United States Food and Drug Administration (FDA) has approved two robotic devices, the Ion™ Endoluminal System (“Ion”) (Intuitive Surgical©, Sunnyvale, CA, USA) and the Monarch robotic system (Auris Health Inc, Redwood City, CA), for peripheral navigation and biopsy of lung lesions. We review these two robotic bronchoscopy systems and the literature that supports their use.
Introduction
Lung cancer is the leading cause of cancer-related mortality in men and women throughout the world, with primary bronchogenic cancers accounting for 23% of all cancer deaths1,2. The 5-year survival of patients with metastatic cancer at diagnosis is only 6.3%, making early detection and diagnosis of pulmonary nodules critical3 With recent advancements in robotic bronchoscopy for peripheral pulmonary nodule biopsy, there has been notable improvements in reach, stability, and precision. Since 2017, the United States Food and Drug Administration (FDA) has approved two robotic devices for peripheral navigation and biopsy of lung lesions4,5. We review these two robotic bronchoscopy systems and the literature that supports their use.
History of Bronchoscopy and Role in Lung Cancer
In 1876, Gustav Killian, the father of modern-day bronchoscopy, extracted a piece of bone from the right bronchus6. Killian later coined the term “directe bronkoscopie” to describe this technique. The next hundred years ushered in a new wave of technology with the development of the rigid bronchoscope, endobronchial cryotherapy, CO2 laser, and endobronchial electrosurgery. Notably, in 1966, Shigeto Ikeda presented the first flexible fiberoptic bronchoscope which revolutionized bronchoscopy6.
As the landscape of lung cancer diagnosis changed with the adoption of screening CT scans, bronchoscopic interventions became an ideal, minimally-invasive modality for sampling concerning lesions with lower complication rates, the ability to sample the lesion and stage the mediastinum in a single procedure, and the attainment of large tissue volume for molecular analysis7,8.
Development of virtual bronchoscopy (VB), radial endobronchial ultrasound (r-EBUS) and electromagnetic navigation (EMN), and/or cone-beam CT (CBCT)-guided bronchoscopy have improved the diagnostic yield of sampling of peripheral pulmonary lesion but have still trailed the diagnostic potential of image-guided transthoracic needle aspiration, reported as high as 96.8%9. The lower diagnostic yield could be explained by lack of direct visualization of the airways and respiratory motion impacting stability during biopsy accuracy, as well as difficulty maintaining catheter position as tools are interchanged.
Development of Robotic Platforms
Two commercially available robotic platforms have been approved which allow direct visualization during navigation, allowing for travel further in the peripheral airways (Table 1). In cadaveric models, robot-assisted bronchoscopy (RAB) was shown to have improved reach in the periphery of the lung in all segments when compared with 4.2 mm OD conventional thin bronchoscopes, particularly in bronchi with increased angulation10. In the PRECISION study, the rate of successful peripheral pulmonary nodule localization and puncture was superior when using robotic bronchoscopy compared with EMN (80% vs 45%; P = .02)11.
Table 1: Comparison of Key Characteristics of the Robotic Platforms
|
|
Ion Robotic Bronchoscopy System |
Monarch Robotic Bronchoscopy System |
|
Technology Type |
Shape-sensing |
Electromagnetic Navigation |
|
Catheter Diameter |
3.5 mm |
4.2 – 6mm |
|
Working Channel |
2 mm |
2.1 mm |
|
Need for Pre-op CT scan |
Yes |
Yes |
|
Need for Patient sensors |
No |
Yes |
|
Need for Room Mapping |
No |
No |
|
Vision During Navigation |
Yes |
Yes |
|
Vision During Biopsy |
No |
Yes |
|
Cone beam compatible |
Sometimes |
Sometimes |
Diagnostic yield has also been improved with RAB compared to prior bronchoscopic technology with ranges from 69.1 to 88%12-14. This may be partially due to decreased deflection during biopsy. Combination with other technologies, like CBCT, is possible with robotic bronchoscopy. The most common ancillary imaging technique used with advanced bronchoscopy is fluoroscopy. In a large prospective study, NAVIGATE, 91% of US participating sites utilized fluoroscopy with 57.4% using radial EBUS. In Europe, 41.7% of sites used fluoroscopy with 4% using radial EBUS15,16. Current trials are underway assessing the impact these complementary devices can have on diagnostic yield with RAB.
In addition to increased efficiency, RAB has also been shown to be safe. Pneumothorax rates in RAB have been reported to be as high as 3.8% compared to 25.9% for CT-guided biopsy12-14,17-23. Minor airway bleeding has been reported with published strategies to approach these situations24. There are currently no reported deaths reported to the FDA associated with RAB.
The Ion™ Endoluminal System
Our institution utilizes the Ion™ Endoluminal System (“Ion”) (Intuitive Surgical©, Sunnyvale, CA, USA) for bronchoscopic sampling of peripheral pulmonary nodules (Figure 1). The Ion procedure uses the PlanPoint Planning Station which incorporates a thin slice preoperative CT scan and generates a 3D airway tree that showcases the navigation pathway to the target with anatomy borders defined (Figure 2). The 3.5 mm (outer diameter) catheter can articulate 180° in any direction to reach any segment of the lung under visualization by the 120° field of view from Ion’s vision probe. The Ion’s thin, flexible fiber optic shape sensor allows the measurement of the full shape of the catheter, providing real-time precise location and shape information throughout the navigation and biopsy process. Unlike electromagnetic navigation (EMN), shape-sensing technology is not subject to interference from nearby metal objects. Additionally, there is no need for an electromagnetic generator, patient sensors, or room mapping. Biopsies are obtained by forceps or a proprietary needle through the 2 mm working channel.
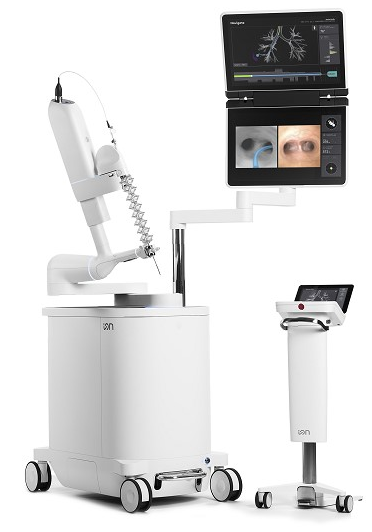
Figure 1: Ion™ Endoluminal System (“Ion”) (Intuitive Surgical©, Sunnyvale, CA, USA)
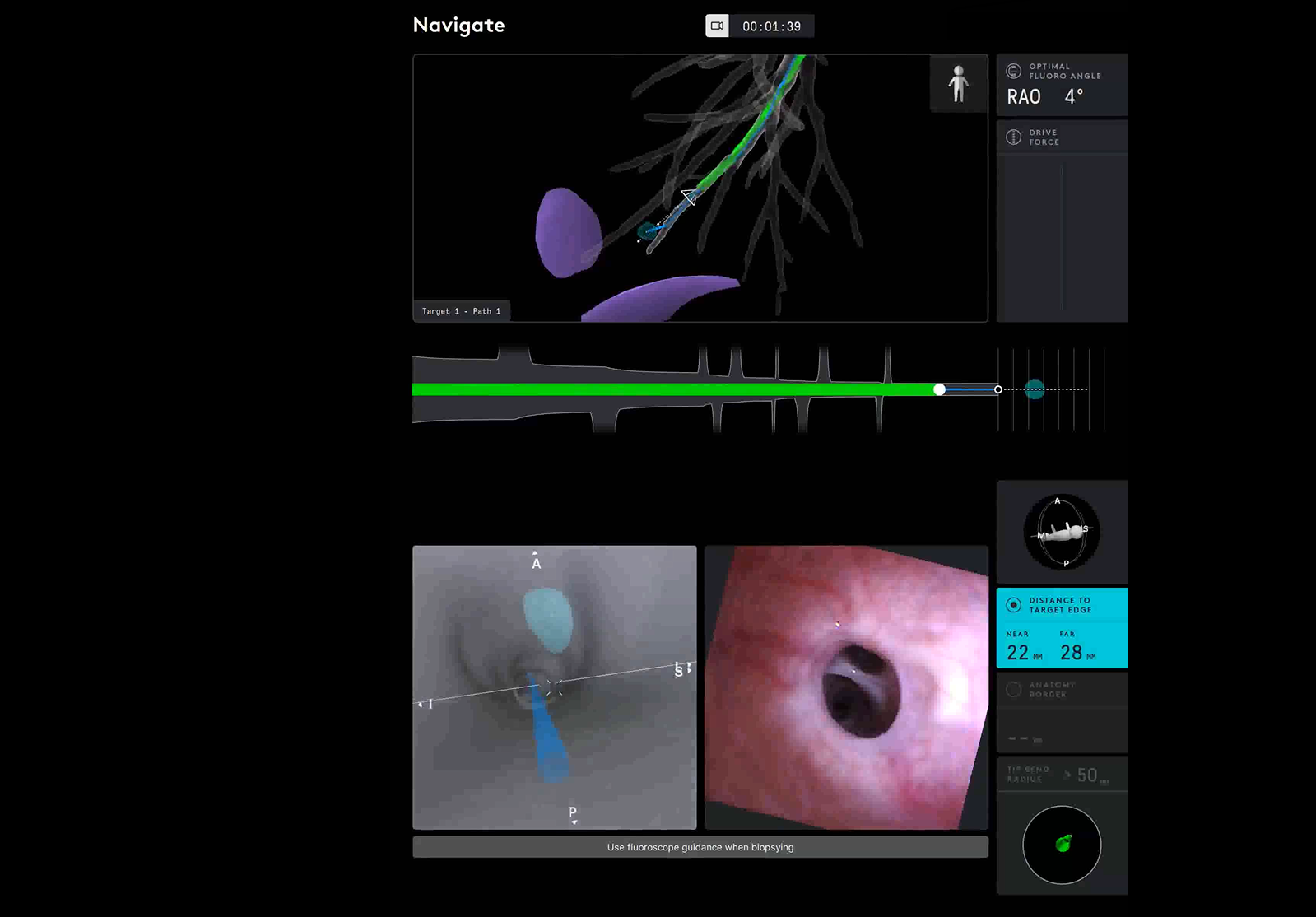
Figure 2: Ion user interface showcasing pathway and distance to target.
The Ion has shown promising results in recent trials. Diagnostic yields from the platform alone have ranged from 81.7 - 88% (13,14,20) (Table 2). Additionally, the shape-sensing robotic-assisted bronchoscopy (SSRAB) has been combined with several other modalities including EBUS, CBCT, and portable 3D imaging. Kalchiem-Dekel et al. performed a prospective study of 131 patients combining fluoroscopy and rEBUS with SSRAB (2D fluoroscopy 79.9%, 3D fluoroscopy 20.1%, rEBUS 85.5%) with a 81.7% diagnostic yield20. The combination of CBCT with SSRAB has also shown improved diagnostic yield of 84 - 94.1%25,26.
Table 2: Diagnostic outcomes of the Ion Robotic Bronchoscopy System
|
Publication |
Sample Size |
Description |
Diagnostic Yield |
Pneumothorax Rate |
|
Fielding 2019 |
N = 29 |
This study sought to evaluate the safety and feasibility SSRAB system. The target was reached in 96.6% of cases. |
79.3% |
0% |
|
Benn 2021 |
N = 52 |
This study demonstrated that successful navigation was completed in 85% of lesions and repositioning accomplished in 15% with adjunctive cone-beam CT. |
86% |
3.8% |
|
Kalchiem-Dekel 2021 |
N = 131 |
This study evaluated 2 dimensional versus 3 dimensional versus radial EBUS adjuncts to the outcomes of robotic bronchoscopy. |
81.7% |
1.5% |
|
Ost 2021 |
N = 34 |
This study is a prospective evaluation of the clinical utility of Ion with CBCT. |
94% |
5.8% (n = 2) |
|
Reisenauer 2021 |
N = 241 |
This study concludes that the novel shape sensing technology has potential to positively impact yields and other outcomes for peripheral robotic bronchoscopy. |
NR |
3.3% |
|
Bajwa 2021 |
N = 76 |
Abstract submission of Ion combined with rEBUS |
92% |
0% |
The Monarch Robotic System
The Monarch robotic system (Auris Health Inc, Redwood City, CA) combines three distinct navigation technologies – electromagnetics, optical pattern recognition and robotic kinematic data – to navigate a catheter to the nodule via a pathway directed by a preprocedural CT (Figure 3). The computer vision utilizes key structures and updates in real-time, maintaining position through lung movement, while calculating catheter depth, articulation angles and distance to target (Figure 4). The Monarch uses an electromagnetic field generator and reference sensors on the patient’s chest to triangulate bronchoscope location to the target lesion via a 6 mm catheter that can be articulated up to 130° in any direction and advancement of the 4.2 mm bronchoscope that can be further extended into the periphery with an additional 180° of flexion in any direction. Biopsies are taken via a 2.1 mm working channel via third-party needles, forceps, and brushes.

Figure 3: Monarch Robotic System (Auris Health Inc, Redwood City, CA)
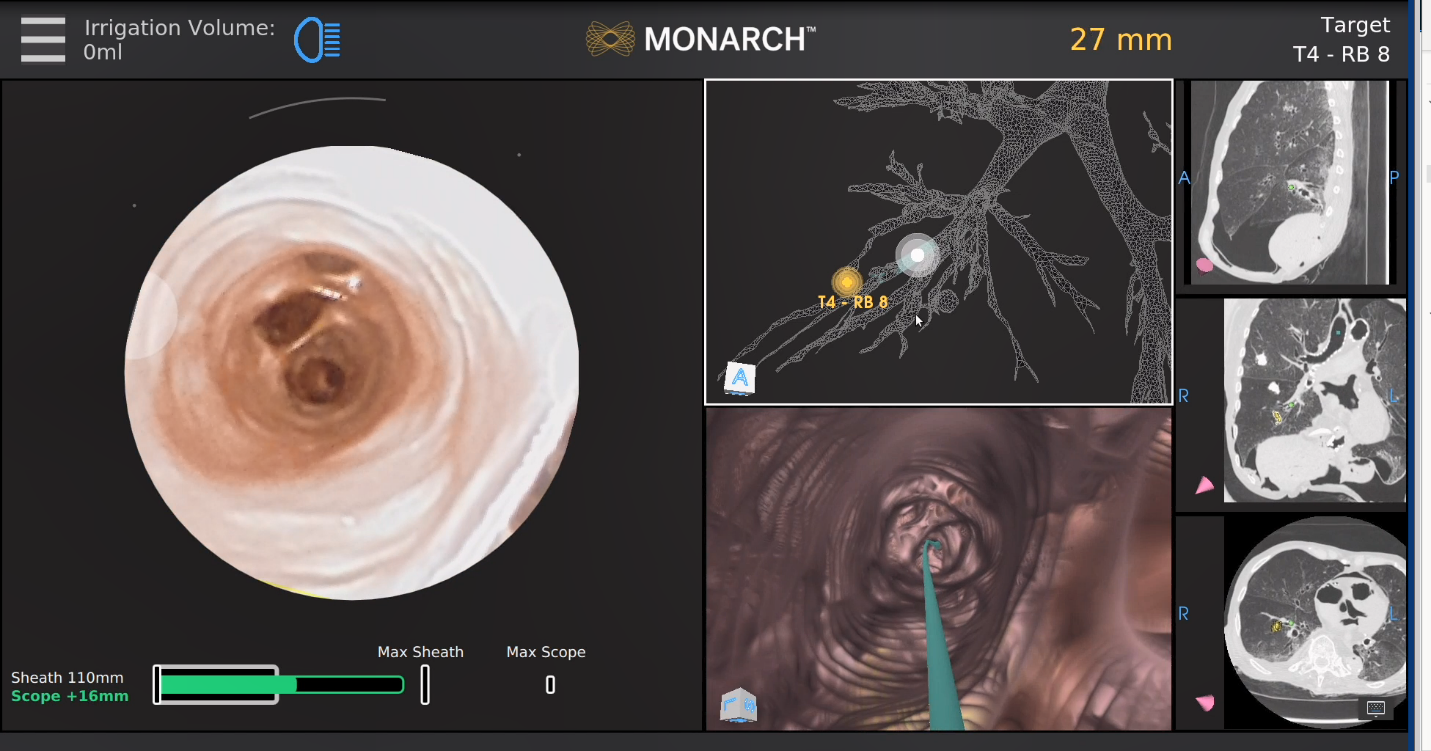
Figure 4: Monarch user interface indicating pathway to target
Multiple multicenter publications centered around the Monarch platform do demonstrate safety and feasibility of the system (Table 3). In 2018, Rojas Solano et al. preformed the first feasibility study with the Monarch system with a nodule localization rate of 93% in 15 patients22. The largest multicenter study with the Monarch platform included 167 lesions with a successful navigation rate of 88.6% and a diagnostic yield of 69-77%. Bleeding was reported in 2.4% of cases and a pneumothorax rate of 3.6%12. Most recently, a prospective, single center study of 124 patients reported a diagnostic yield of 77% and a bleeding rate of 3.2%27.
Table 3: Diagnostic outcomes of the Monarch Robotic Bronchoscopy System
|
Publication |
Sample Size |
Description |
Diagnostic Yield |
Pneumothorax Rate |
|
Rojas Solano 2018 |
N = 15 |
Feasibility study performed at a single center with localization rate of 93% |
NR |
0% |
|
Chaddha 2019 |
N = 167 |
A multicenter study with the Monarch platform with a navigation rate of 88.6%. Bleeding reported in 2.4% of cases. |
69–77% |
3.6% |
|
Chen 2020 |
N = 55 |
A prospective, multicenter study of the Monarch system. Localization was achieved in 96.2%. |
69-77% |
3.7% |
|
Ekeke 2021 |
N = 25 |
A retrospective review of robotic-assisted bronchoscopy with EMN and biopsy. |
96% |
0% |
|
Agrawal 2022 |
N = 124 |
Prospective, single center study analyzing factors affecting diagnostic accuracy and provide 12-month follow-up. Bleeding rate of 3.2% |
77% |
1.6% |
Future Directions: The Galaxy System
In addition to the two currently available robotic bronchoscopy systems, Noah Medical is developing an EMN-based system called The Galaxy System (Noah Medical, San Carlos, CA). This system boasts a smaller footprint than the other two systems with TiLT⺠integrated digital tomosynthesis that can be performed using a C-arm as well as capability for augmented fluoroscopy, theoretically obviating the need for integration of an advanced imaging system. Combining intraprocedural tomosynthesis-based technology may enhance lesion visibility and improve CT-to-body divergence with an integrated local registration feature that updates the relationship between the catheter and the lesion28. Additionally, the system offers a single-use disposable bronchoscope, reducing cross contamination and the overall burden of reprocessing a reusable scope.
Limitations of Robotic-assisted bronchoscopy
Despite the exciting developments in nodule diagnosis using robotic-assisted bronchoscopy, there are several limitations in the field that will need to be addressed. Despite the growing literature in diagnostic yield and associated complications, there has yet to be a randomized, controlled trial comparing the gold-standard of CT-guided biopsy to RAB. Additionally, the different robotic systems differ in the technology employed for navigation; no trial has compared the two systems performance directly. Finally, many of the studies analyzing the RAB systems have primary endpoints in diagnostic yield; however, other information such as tissue for molecular markers has not been assessed as an important parameter.
Conclusion
The advent of two robotic-assisted platforms has shepherded in new possibilities for navigation bronchoscopy and the diagnosis and treatment of lung cancer. While the technologies of these systems differ, their improvement in reach, stability, and precision seen in initial published evidence provide a foundation to build future bronchoscopic applications for these lesions.
Conflict of Interest
The authors have no conflicts of interest to declare.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019; 144(8): 1941-53.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68(1): 7-30.
- Institue NC. Cancer Stat Facts: Lung and Bronchus Cancer. Surveillance, Epidemiology, and End Results Program 2021. Available from: https://seer.cancer.gov/statfacts/html/lungb.html
- Administration USFaD. 501(k) Premarket Notification - Monarch Endoscopy Platform (Report K173760) 2018. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K173760
- Administration USFaD. 510(k) Premarket Notification - Ion Endoluminal System. (Report K192367). 2019.
- Panchabhai TS, Mehta AC. Historical perspectives of bronchoscopy. Connecting the dots. Ann Am Thorac Soc. 2015; 12(5): 631-41.
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018; 16(7): 807-21.
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; 143(5 Suppl): e93S-e120S.
- Loh SE, Wu DD, Venkatesh SK, et al. CT-guided thoracic biopsy: evaluating diagnostic yield and complications. Ann Acad Med Singap. 2013; 42(6): 285-90.
- Chen AC, Gillespie CT. Robotic Endoscopic Airway Challenge: REACH Assessment. Ann Thorac Surg. 2018; 106(1): 293-7.
- Yarmus L, Akulian J, Wahidi M, et al. A Prospective Randomized Comparative Study of Three Guided Bronchoscopic Approaches for Investigating Pulmonary Nodules: The PRECISION-1 Study. Chest. 2020; 157(3): 694-701.
- Chaddha U, Kovacs SP, Manley C, et al. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience. BMC Pulm Med. 2019; 19(1): 243.
- Fielding DIK, Bashirzadeh F, Son JH, et al. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration. 2019; 98(2): 142-50.
- Ost D PM, Reisenauer JS, et al. Prospective multicenter analysis of shape-sensing robotic-assisted bronchoscopy for the biopsy of pulmonary nodules: results from the PRECISE study. 2021.
- Folch EE, Bowling MR, Pritchett MA, et al. NAVIGATE 24-Month Results: Electromagnetic Navigation Bronchoscopy for Pulmonary Lesions at 37 Centers in Europe and the United States. J Thorac Oncol. 2021.
- Khandhar SJ, Bowling MR, Flandes J, et al. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study. BMC Pulm Med. 2017; 17(1): 59.
- Benn BS, Romero AO, Lum M, et al. Robotic-Assisted Navigation Bronchoscopy as a Paradigm Shift in Peripheral Lung Access. Lung. 2021; 199(2): 177-86.
- Chen AC, Pastis NJ, Jr., Mahajan AK, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest. 2021; 159(2): 845-52.
- Huo YR, Chan MV, Habib AR, et al. Pneumothorax rates in CT-Guided lung biopsies: a comprehensive systematic review and meta-analysis of risk factors. Br J Radiol. 2020; 93(1108): 20190866.
- Kalchiem-Dekel O, Connolly JG, Lin IH, et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest. 2022; 161(2): 572-82.
- Reisenauer J, Simoff MJ, Pritchett MA, et al. Ion: Technology and Techniques for Shape-sensing Robotic-assisted Bronchoscopy. Ann Thorac Surg. 2022; 113(1): 308-15.
- Rojas-Solano JR, Ugalde-Gamboa L, Machuzak M. Robotic Bronchoscopy for Diagnosis of Suspected Lung Cancer: A Feasibility Study. J Bronchology Interv Pulmonol. 2018; 25(3): 168-75.
- Simoff MJ, Pritchett MA, Reisenauer JS, et al. Shape-sensing robotic-assisted bronchoscopy for pulmonary nodules: initial multicenter experience using the Ion Endoluminal System. BMC Pulm Med. 2021; 21(1): 322.
- Fernandez-Bussy S, Abia-Trujillo D, Majid A, et al. Management of Significant Airway Bleeding during Robotic Assisted Bronchoscopy: A Tailored Approach. Respiration. 2021; 100(6): 547-50.
- Benn BS, Romero AO, Bawaadam H, et al. Cone Beam CT Guidance Improves Transbronchial Lung Cryobiopsy Safety. Lung. 2021; 199(5): 485-92.
- Pritchett M, Schirmer C. Shape-sensing robotic assisted bronchoscopy for the diadnosis of peripheral pulmonary lesions. CHEST. 2021; 160(4): 1631-32.
- Agrawal A, Ho E, Chaddha U, et al. Factors Associated with Diagnostic Accuracy of Robotic Bronchoscopy with 12-month Follow-up. Ann Thorac Surg. 2022.
- Pritchett MA, Bhadra K, Mattingley JS. Electromagnetic Navigation Bronchoscopy With Tomosynthesis-based Visualization and Positional Correction: Three-dimensional Accuracy as Confirmed by Cone-Beam Computed Tomography. J Bronchology Interv Pulmonol. 2021; 28(1): 10-20.
