Pneumatosis Cystoides Intestinalis
Yuusaku Sugihara1, 2*, Keita Harada1, Hiroko Ogawa2, Fumio Otsuka2, Hiroyuki Okada1
1Department of Gastroenterology and Hepatology, Okayama University Hospital, 2-5-1 Shikata-cho, Kita-ku, Okayaka-shi, Okayama 700-8558, Japan
2Department of General internal medicine, Okayama University Hospital, 2-5-1 Shikata-cho, Kita-ku, Okayaka-shi, Okayama 700-8558, Japan
Abstract
Pneumatosis cystoides intestinalis (PCI) is the presence of air within the walls of the small intestines or colon. The prevalence of PCI is difficult to determine as patients are mostly asymptomatic. The main cause of PCI is considered to be mechanical, although bacterial and biochemical causes have also been theorized. According to the mechanical theory, PCI results from a loss of integrity of the mucosa, caused by diseases such as necrotizing enterocolitis, intestinal ischemia, inflammatory bowel disease, or obstructive pulmonary disease. On computed tomography, PCI presents as images that are cystic or linear collections of air near the lumen of the bowel. The occurrence of PCI is more common in the colon than in the small intestine, appearing as a polypoid, with the overlying mucosa displaying a blue hue on colonoscopy. PCI can usually be resolved through the discontinuation of medications that increase the formation of intestinal gas. Inhalational and hyperbaric oxygen therapy have also been used for the treatment of PCI. Patients presenting with signs of peritonitis, such as abdominal guarding, rebound tenderness, and metabolic acidosis on abdominal assessment, require emergent treatment, with exploratory laparotomy being recommended.
INTRODUCTION
Pneumatosis cystoides intestinalis (PCI) refers to the presence of air within the wall of the small intestine or colon. This intra-mucosal air can also affect the stomach, a condition known as gastric pneumatosis. Since Du Vernoy first reported PCI in autopsy specimens in 17301, the condition has been described in various studies. PCI occurs as a primary (or idiopathic) form in 15% of cases, and as a secondary form in 85% of cases2. As patients with PCI are mostly asymptomatic, they are not likely to seek medical care. Therefore, the true prevalence of PCI is difficult to determine. A previous study reported an incidence rate of 0.03% in the general population, typically developing in the fifth to eighth decade of life, with a male-to-female ratio of 3:13.
PCI can develop in any portion of the gastrointestinal tract distal to the stomach, with the cysts being either confined to the mucosa, submucosa, or subserosa or involving all three layers. Subserosal cysts develop more commonly with pneumatosis of the small intestine, with submucosal cysts being more common with colonic pneumatosis. On gross inspection, submucosal cysts are polypoid, with the overlying mucosa displaying a bluish hue.
PATHOGENESIS
The pathogenesis of PCI is poorly understood, with multiple contributing pathogenic mechanisms being likely, including mechanical, bacterial and biochemical factors4. Among these, mechanical causes have gained the widest recognition, with the air invading the wall of the bowel secondary to integrity loss of the mucosa or serosa5. This mechanical PCI pathway explains the association between PCI and necrotizing enterocolitis, intestinal ischemia, inflammatory bowel disease, colonoscopy preparation, and colonoscopy. Once air has entered the wall of the bowel, it may spread along the mesentery to distant sites. This spread of air along blood vessels further explains the association between PCI and chronic obstructive pulmonary disease, asthma, and interstitial pneumonia, which can injure the serosa, as well as the rupture of alveolar blood vessels due to coughing. Specifically, escaped air tracks along blood vessels to the mesenteric blood vessels, eventually penetrating the bowel wall6.
In contrast to the mechanical theory of PCI development, the bacterial theory proposes that PCI results from gas-forming bacteria which provide an access from the mucosa to the submucosa, the mechanism of which has been reproduced by injecting gas-forming bacteria into the bowel wall of rats7. Bacterial PCI can be improved with the use of antibiotic treatment. In a similar way, the biochemical theory proposes that luminal bacteria produce excessive amounts of hydrogen gas. As the pressure of the gas within the intestinal lumen increases, it is forced through the mucosa, becoming entrapped within the submucosa. This biochemical type of PCI has been reported in patients with bacterial overgrowth in the small bowel, as well as in patients taking alpha glycosidase inhibitors, which increases intestinal gas.
PCI has also been associated with chemotherapy and hormonal therapy8, as well as with the use of various molecular-targeting agents used for the treatment of malignant tumors9. PCI can also develop over the clinical course of connective tissue disease, particularly systemic sclerosis (SSc). Although the mechanism of SSc-associated PCI has not been fully elucidated, sclerosis of the intestinal wall is likely to be the principal cause, with the resulting impairment in gastrointestinal motility eventually causing intestinal obstruction and, subsequently, increased luminal pressure. Furthermore, there are reports that PCI is caused by mechanical stimulation during laparoscopic procedures10.
CLINICAL FEATURES AND DIAGNOSIS OF PCI
As mentioned above, most patients with PCI are asymptomatic, never requiring clinical treatment. Complications occur in approximately 3% of patients11, owing to encroachment of the lumen by cysts, which eventually results in obstruction of the small and/or large bowel. When symptomatic, PCI can present as abdominal pain, obstruction, diarrhea, and hematochezia, with flatulence, loss of appetite and tenesmus being other common symptoms. The confirmation of PCI using clinical imaging, however, is not straightforward. PCI is only visible on plain abdominal radiographs in about two-thirds of patients. One concern is that stool can mimic the appearance of PCI on plain radiographs. On ultrasound imaging, there was reverberation caused by the presence of air within the cysts. Findings are more characteristic on computed tomography (CT) imaging, with PCI appearing as grape-like clusters or honeycomb-shaped shadows along the wall of the intestine. However, these clusters can be filled with either free or trapped air, with free air not being a cause of the clinical complications of PCI12. Furthermore, there are reports in CT findings of the following observations. Three patterns of pneumatosis have been described: a bubble-like or cystoid pattern characterized by separate bubbles of gas with a cystic appearance; a linear pattern in which the gas has a curvilinear or a crescent shape and a circular or circumferential form in the bowel wall; in some cases, all these patterns may be seen at the same time13. Endoscopic examination can be helpful for differentiating PCI from non-PCI related mucosal and submucosal lesions. Although PCI was once considered as being specific to the small intestine, a recent colonoscopy study reported a more frequent occurrence of colonic PCI14. On endoscopy, PCI is observed as polypoid, with the overlying mucosa displaying a blue hue. Of note, submucosal tumors also have a bluish appearance on endoscopy, making it difficult to clearly differentiate mucosal or submucosal lesions from PCI solely based on endoscopic appearance. A reliable differentiation can be obtained by endoscopic biopsy; however, the puncture of mucosal and submucosal cysts itself can be a cause of PCI.
Laboratory studies can be helpful for distinguishing PCI from other causes of abdominal symptoms. Specifically, laboratory tests are usually normal in patients with PCI, while mesenteric ischemia or bowel infarction are associated with a marked leukocytosis, with a predominance of white blood cells.
MANAGEMENT AND TREATMENT
Emergency treatment is needed for patients with signs of peritonitis on abdominal assessment, such as findings of abdominal guarding, rebound tenderness, or metabolic acidosis. Exploratory laparotomy is recommended for these patients. If the cysts of PCI are ruptured on the serosa side, CT can show free air, but there are patients who do not require emergent treatment14 (Figure). For patients who do not require emergency exploratory laparotomy, management is based on the severity of symptoms, with PCI resolving without intervention in the majority of patients who do not present with significant symptoms. A conservative approach to PCI management is recommended, such as gastrointestinal decompression or intestinal rest. It was reported that most cases could be treated with conservative treatment15. The underlying cause of PCI should be treated, including the discontinuation of medications known to increase intestinal gas formation, such as alpha-glucosidase inhibitors and molecular targeted therapy. The continuation of antibiotic treatment is one of the choices for PCI until clinical and radiological resolution of PCI is achieved. Most commonly, metronidazole (500 mg, three times daily) is used for antibiotic therapy after informing patients of the possible adverse effects of this medication. It is thought that this antibiotic treatment for PCI eradicates gas-forming bacteria. Parenteral nutrition, electrolyte supplementation and the use of an elemental diet have also been reported as possible treatments for PCI, with the effectiveness of these interventions being mediated through a modification of the colonic micro flora.
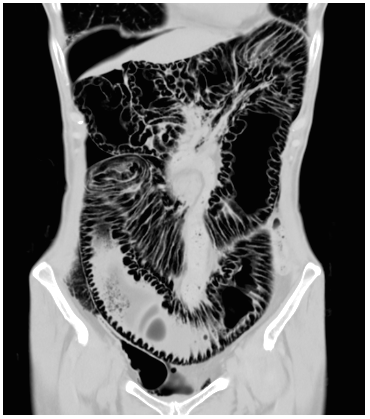
Figure 1: Coronal CT reconstruction scan showed the colon with air in the submucosa and subserosa, and free air under the right hypochondrium.
Inhalational or hyperbaric oxygen therapy has also been used to treat PCI. Although oxygen was first used for the treatment of PCI by Forgacs et al16 in 1973, the optimal concentration, duration, and effect of oxygen have not yet been determined, with the following prescriptions generally recommended for gas absorption within the cysts: 200-300 mmHg PO2 pressure, 1.5-2.5 h/day, for 2-14 days; or 55%-75% oxygen inhalation, 1-3 h/day, for 2-5 days. The basis for the effectiveness of oxygen therapy is twofold. First, oxygen is toxic to the anaerobic intestinal bacteria that contribute to the formation of air within cysts. Secondly, as the content within cysts is primarily non-oxygen gas, delivery of high concentrations of oxygen increases the partial pressure of oxygen in the venous blood, while decreasing the partial pressure of non-oxygen gases, thereby creating a diffusion gradient across the cystic wall and forcing trapped gas to exit from cysts.
Surgery is not always recommended for patients who develop PCI-related intestinal obstruction because of the high operative risk. In such cases, successful relief of obstruction may be achieved using endoscopic puncture and sclerotherapy of cysts17. Surgery, including exploratory laparotomy, should be reserved for patients who remain symptomatic despite medical therapy or who develop PCI-related complications, such as bowel obstruction, perforation, peritonitis, and necrosis. Therefore, early detection of PCI, when possible, and provision of fast and adequate therapy for PCI resolution, are clinically important.
In conclusion, PCI is a rare disorder encountered in clinical practice that is poorly understood. Careful monitoring of patients with PCI is recommended for early identification of emergent signs and symptoms of complication. The absence of a definitive causal theory of PCI and the variety of treatments which have been reported reflect our current lack of knowledge regarding the underlying pathophysiology of PCI. A long-term follow-up study is warranted to evaluate the clinical outcomes of treatments for PCI.
References
- Du Vernoy JG. Aer intestinorum tam sub extima quam intima tunica inclusus: observationes anatomicae: comment. Acad Acient Imp Petropol. 1730; 5: 213-225.
- Feczko PJ1, Mezwa DG, Farah MC, et al. Clinical significance of pneumatosis of the bowel wall. Radiographics. 1992; 12(6): 1069–1078.
- Wu LL, Yang YS, Dou Y, et al. A systematic analysis of pneumatosis cystoides intestinalis. World J Gastroenterol. 2013; 19(30): 4973-4978.
- Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990; 212(2): 160-165.
- Pieterse AS, Leong AS, Rowland R. The mucosal changes and pathogenesis of pneumatosis cystoides intestinalis. Hum Pathol. 1985; 16(7): 683-688.
- Keyting WS, Mccarver RR, Kovarik JL, et al. Pneumatosis intestinalis: a new concept. Radiology. 1961; 76(5): 733-741.
- Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg. 1974; 109(1): 89-94.
- Groninger E, Hulscher JB, Timmer B, et al. Free air intraperitoneally during chemotherapy for acute lymphoblastic leukemia: consider pneumatosis cystoides intestinalis. J Pediatr Hematol Oncol. 2010; 32(2): 141-143.
- Flaig TW, Kim FJ, La Rosa FG, et al. Colonic pneumatosis and intestinal perforations with sunitinib treatment for renal cell carcinoma. Invest New Drugs. 2009; 27(1): 83–87.
- Castren EE, Hakeem AR, Mahmood NS, et al. Case of pneumatosis intestinalis and hepatic portal venous gas following a laparoscopic right hemicolectomy. BMJ Case Rep. 2016: bcr2016214431.
- Galandiuk S, Fazio VW. Pneumatosis cystoides intestinalis. A review of the literature. Dis Colon Rectum. 1986; 29(5): 358-363.
- Horiuchi A, Akamatsu T, Mukawa K, et al. Case report: Pneumatosis cystoides intestinalis associated with post-surgical bowel anastomosis: a report of three cases and review of the Japanese literature. J Gastroenterol Hepatol. 1998; 13(5): 534-537.
- Lassandro F, Valente T, Rea G, et al. Imaging assessment and clinical significance of pneumatosis in adult patients. Radiol Med. 2015; 120: 96-104.
- Sugihara Y, Okada H. Pneumatosis cystoides intestinalis. N Engl J Med. 2017; 377: 2266.
- Wu LL, Yang YS, Dou Y, et al. A systematic analysis of pneumatosis cystoids intestinalis. World J Gastroenterol. 2013; 19(30): 4973-4978.
- Forgacs P, Wright PH, Wyatt AP. Treatment of intestinal gas cysts by oxygen breathing. Lancet. 1973; 1(7803): 579-582.
- Johansson K, Lindström E. Treatment of obstructive pneumatosis coli with endoscopic sclerotherapy: report of a case. Dis Colon Rectum. 1991(34); 34: 94-96.
