Exhaled breath condensate: a potential tool to evaluate the relationship between inflammation and dyspnea in patients with chronic obstructive pulmonary disease
Stephanos Patsiris1,2*, Grigoris Stelios2, Ilias Papanikolaou1, Themis Exarchos2, Panayiotis Vlamos2
1General Hospital of Corfu, Corfu, Greece
2Bioinformatics & Human Electrophysiology Laboratory, Dept. of Informatics, Ionian University, Corfu, Greece
Abstract
Chronic obstructive pulmonary disease (COPD) is a respiratory disease with high prevalence. Many factors contribute to its development, and probably that leads to its various clinical pictures. Inflammation is the mechanism responsible for the structural alterations in the lungs. Despite its heterogeneity, there are a couple of primary symptoms characterizing it, which are chronic and productive cough and dyspnea.
The understanding of dyspnea in COPD is based on theories deriving from the interaction of a network formed between the cardiorespiratory and the neuromuscular system and their receptors. Many factors contribute to its occurrence, making it complex and giving it a very subjective character for a person to perceive.
Various methods are used to study COPD. Non-invasive ones seem to attract attention nowadays. One of them is the exhaled breath condensate. It is a biofluid with rich content, which can capture a picture of the pathological processes happening in the lungs. Its study has shown that some markers of inflammation and oxidative stress, such as 8-isoprostane and H2O2, are elevated and able to connect dyspnea and inflammation. Additionally, they seem to provide information of the ongoing inflammatory process in the lungs as well as a picture of the severity of the symptoms. This evidence may enhance the association of dyspnea with dysfunctional breathing.
Despite these interesting findings, further research is necessary both in dyspnea and inflammation in COPD to clarify their mechanisms and connective pathways. The utility of non-invasive techniques such as the exhaled breath condensate could be of significant help, but its establishment in the medical field requires extra studies.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic health problem with high morbidity and mortality1,2. The Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) defines it as a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases3 It is a complex heterogenous condition that has also been described as a syndrome4. Its etiology is multifactorial with smoking remaining the primary cause. Inflammation possesses a key role in the development of chronic obstructive pulmonary disease resulting in structural changes in the lungs5. Although it presents a variety in clinical manifestations, there are few cardinal symptoms that describe it and these are dyspnea and chronic and productive cough6. Especially, dyspnea is the reason that will lead a person to seek medical help.
According to the American Thoracic Society, dyspnea is the unpleasant symptom of the subjective experience of breathing discomfort resulting from qualitatively distinct sensations that vary in intensity7. There are multiple factors that play a role in its complex mechanism, such as physiologic, psychological and environmental and they determine the degree of occurrence and perception. Dyspnea can be either acute or chronic8. Experimental models of dyspnea have been developed trying to elucidate its various mechanisms. Our current understanding of dyspnea is expressed by the theory of a result of the collection and interpretation of mechanical and chemical information that derives from a network between the cardiorespiratory system and the neuromuscular system9.
Dyspnea is COPD activity–related and the progression of the disease leads to an established and constant feeling of lack of air. It is believed that it is the result of disruption in the normal relationship of the inspiratory neural drive to breathe and the dynamic response of the respiratory system. An increase in the respiratory neural drive is observed in COPD, which seems to be correlated to abnormalities in pulmonary gases exchange, imbalance in acid–base equilibrium and disorder in dynamic respiratory mechanics10. The awareness of this respiratory distress symptom is expressed in the form of work/effort, tightness and air hunger, a fact that relies on pathways and distinct mechanisms of sensory qualities11.
The dyspnea issue and its occurrence in COPD, especially in the form of activity-related, still require a better understanding regarding its mechanisms. The pathological processes blamed for the development of COPD, such as inflammation and oxidative stress are not included in the explanation of dyspnea in COPD so far. However, the study of the pathology of COPD via noninvasive techniques such as the exhaled breath condensate, has presented data of the potential connection between dyspnea and inflammation.
Exhaled breath condensate
The exhaled breath condensate is a biofluid and is formed by freezing the exhaled breathing air. Nowadays, it is strongly believed to originate mainly from the small airways and the alveola. It is a cocktail of substances with water being the predominant one (99%). The rest of the content is a mix of volatile organic compounds and non-volatile organic compounds covering a range from small inorganic ions to large and macromolecules even proteins and peptides. The exhaled breath condensate reflects the composition of the airway lining fluid, a fact that seems important considering that the small airways and lung parenchyma are mainly affected in COPD12,13.
As a technique has many advantages and the most significant ones are its non-invasive nature, an application without age limit, no restriction of disease stage (it can be used even with intubated patients) and of course, the pure nature of the collected product without any effect from external intervention. Its potential usage has a wide spectrum covering areas such as diagnosis, early detection, monitoring, phenotype discrimination, treatment response, and follow-up14.
Pictures of the ongoing pathological procedures were obtained by studying COPD via the application of the exhaled breath condensate. The detected changes are mirrored through the alterations in inflammatory markers and mediators found in exhaled breath condensate and provide information about abnormal activity in the lungs. These substances belong to various groups of compounds and can be nitrogen reactive species, cytokines, prostaglandins, leukotrienes and reactive oxygen species. The identified ones have reached the number of 200015. Exhaled breath condensate pH is also a variable showing the acidification of airways that is postulated to be associated with inflammation and play a role in COPD16. Among these elements, the ones extensively analyzed and which seem to have a predominant role in the pathophysiology of the lungs are hydrogen peroxide (H2O2), 8-isoprostane, leukotriene B4 (LTB4), cytokines, prostaglandins E2 (PGE2) and nitrogen oxides (NOx). Probably different elements of EBC reflect different aspects of the inflammatory process17.
Generally, the collection of the exhaled breath condensate can be performed with various devices that freeze the exhaled breath. However, today there are commercial sampling devices that have been tested for this reason and provide reliable results. Each of them has its technical characteristics and parameters to be set before or during the collection process such as time18. Its analysis, on the other hand, seems to be not an easy task. Many methods are used to achieve identification and quantification of the content of the exhaled breath condensate and present variation. Many factors determine which method to be applied such as the determination of a specific compound. Most of them are based on spectrophotometric, fluorometric assays or high-pressure liquid chromatography and are accompanied by the appropriate detection methods19.
Inflammation and dyspnea in COPD
The key feature for the development of COPD is inflammation. It involves groups of cells that functionally exaggerate leading to impairment in the airways. It seems to be an orchestrated process with macrophages, neutrophils, lymphocytes (T and B) and dendritic cells ruling it (mainly in the stable stage)20. Their action is accompanied by mediators necessary for the activation of signaling pathways. Some of them are the reactive oxygen and nitrogen species and their concentration imbalance causes oxidative stress. Oxidative stress is an additional driving mechanism that is engaged in the pathogenesis of COPD with a significant role21. Both cells and mediators via their properties and function, transform the condition into chronic and result in pathological abnormalities such as airway remodeling, mucous plugging and immune cell infiltration22. The structural and functional changes in the airways of the patients with COPD are expressed with chronic and productive cough and dyspnea.
Dyspnea is believed to be not a single sensation and has a multidimensional nature. Its intensity varies and its perception is largely self-reported making its accurate quantification inadequate23,24. Forced expiratory volume in 1 second (FEV1) as the golden standard tool for COPD diagnosis is not capable of being the best physiological variable of dyspnea measurement. The evaluation gap is replaced by the utility of other assessment tools such as questionnaires and scales, which rely on perception ability25.
Little is known about the association between dyspnea and inflammation in COPD, perhaps because of its study as a secondary subject. A source that provides some information and a potential correlation between them is the exhaled breath condensate. Substances of its content present alterations in concentration in both stable and exacerbated stages of COPD. Two well studied examples are 8-isoprostane and hydrogen peroxide (H2O2), which are mediators of oxidative stress26.
8-isoprostane is a member of the F2 isoprostane class and is produced in the phospholipid membranes by free radical-catalyzed peroxidation of arachidonic acid. It is an in vivo formation with a chemical stable character and specific for lipid peroxidation. It belongs to the important steps of oxidative stress. Moreover, it possesses biological activity which is presented by inducing vasoconstriction, contraction of smooth muscles of large and small airways and augmentation in the perception of pain27.
H2O2 is a small volatile inorganic biomolecule that belongs to reactive oxygen species. It is a marker of pulmonary inflammation and oxidative stress, produced exogenously and endogenously by both active inflammatory cells (macrophages, neutrophils, eosinophils) and epithelial cells through the metabolism of superoxide anion by superoxide dismutase (SOD). Its low molecular weight allows it to transfer to the extracellular spaces through the membranes. It is characterized as a stable marker but less than 8-isoprostane28.
The concentration of 8-isoprostane and H2O2 present elevation in the exhaled breath condensate of patients with COPD29. This evidence supports the existence of oxidative stress, which is responsible for the damage especially of the lower respiratory tract. Moreover, the estimation of these to mediators contributes to the evaluation of the severity of the airway inflammation in COPD. This seems to provide a picture of the underlying pathological processes taking place in the airways and parenchyma of the lungs30,31. The biological monitoring of such events taking place especially in the small airways in COPD is crucial as it will help in understanding its spectrum as a disease.
Regarding dyspnea (Figure 1), a few studies have shown that inflammation correlates well with it, resulting from the evaluation of most assessment tools of dyspnea such as mMRC (modified medical research council), CAT (COPD assessment test) and BODE index (Body – mass index, airflow Obstruction, Dyspnea & Exercise) and the elevated levels of H2O2 and 8-isoprostane in exhaled breath condensate of patients with COPD32,33,34. The Cavalcante et al.35 study supported this correlation as they managed to improve dyspnea of patients with COPD by reducing the 8-isoprostane concentration with medication. Generally, these studies showed that symptomatic severity and health status can be assessed by studying 8-isoprostane and H2O236,37.
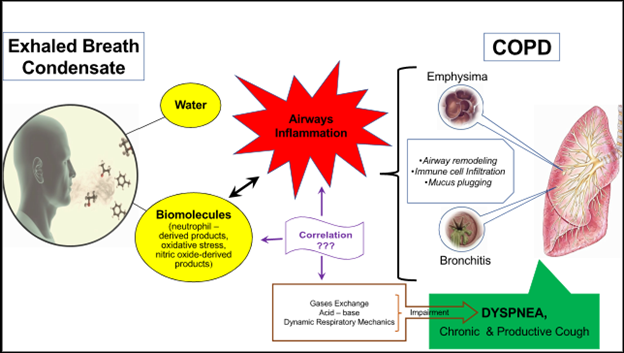
Figure 1: Exhaled breath condensate for the evaluation of the potential association of inflammation and dyspnea in COPD
Although the mechanism of their association is not clear, a possible explanation is that both mediators can be used as markers to assess oxidative stress and inflammation. Particularly of oxidative stress, which is responsible for lung tissue injury because it amplifies inflammation, leads to damage and apoptosis of the airway lining epithelium cells, DNA damage and stimulates emphysema and fibrosis. It may also cause bronchial hyperresponsiveness and mucus gland hyperplasia38.
There is also one report that associates 8-isoprostane and H2O2 with the functional alterations of the musculoskeletal thoracic cage observed in patients with COPD and dyspnea. Specifically, increased levels of 8-isoprostane in the exhaled breath condensate of patients at a stable stage of COPD are related to dynamic hyperinflation according to Garci-Rio et al.39. It also seems that the degree of emphysema is associated with 8-isoprostane concentration measured with high-resolution computed tomography referred by Makris et al.40.
An additional related assumption derives from the connection of the airway nerves and inflammation that leads to the dyspneic sensation. Lungs have vagal receptors which respond to chemical and mechanical stimuli. The inflammatory environment in COPD affects the vagal sensory nerve activity via the stimulation of a group of vagal receptors such as C-fibers and irritant receptors. Irritant receptors are located around the epithelial cells of the bronchial walls while C-fibers innervate the airways and the lungs 41. It has been postulated that oxidative stress causes a perturbation to these receptors that are capable to evoke dyspnea42,43. However, this has not been assessed by studying the EBC.
It is worth mentioning that despite the above interesting findings, the exhaled breath condensate has not got an established place in clinical practice. The main reason is the lack of standardization. A significant effort was made by the European Respiratory Society in 2017 with a report of technical standards44 to minimize the biases and controversies but still, more research is required.
Conclusion
Dyspnea is the cardinal symptom of COPD. Its mechanism of development and perception relies on evidence of interaction between the cardiorespiratory and the neuromusculoskeletal system. However, non-invasive techniques for measuring inflammation such as the exhaled breath condensate have shown a possible association between dyspnea and inflammation in COPD patients. Particularly, mediators involved in inflammation and oxidative stress such as 8-isoprostane and H2O2 seem to correlate well with dyspnea estimated with most assessment dyspnea tools. Their elevated concentration might be related to functional (such as hyperinflation) and structural changes (emphysema) as well.
Further research is necessary in dyspnea in COPD in order to reach a full understanding of that symptom. The utility of non-invasive tools such as the exhaled breath condensate seems to be able to contribute to that and probably provide additional information in the mechanism of dyspnea as it approaches the issue via direct study of the pathological processes that take place within the airways.
References
- Zhang WZ, Gomi K, Mahjour SB, et al. Update in Chronic Obstructive Pulmonary Disease 2017. Am J Respir Crit Care. 2018; 197(12): 1534-1539.
- Duffy SP, Criner GJ. Chronic Obstructive Pulmonary Disease: Evaluation and Management. Med Clin North Am. 2019; 103(3): 453-461.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2020 report. GOLD, 2019. Available at: org/gold-reports/
- Roche N. Adding biological markers to COPD categorisation schemes: a way towards more personalized care? Eur Respir J. 2016; 47(6): 1601-1605.
- Wang Y, Xu J, Meng Y, et al. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obstruct Pulmon Dis. 2018; 13: 3341-3348.
- Reyes-Garcia A, Torre-Bouskoulet L, Perez-Padilla R. Controversies and limitations in the diagnosis of chronic obstructive pulmonary disease. Rev Invest Clin. 2019; 71(1): 28-35.
- Parshall MB, Schwartzstein RM, Adams L, et al. An Ofï¬cial American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea. American Journal of Respiratory & Critical Care Medicine. 2012; 185(4): 435-452.
- Berliner D, Schneider N, Welte T, et al. The differential diagnosis of dyspnea. Dtsch Arztebl Int. 2016; 113(49): 834-845.
- Hanania NA, O’Donnell DE. Activity – related dyspnea in chronic obstructive pulmonary disease: physical and psychological consequences, unmet needs, and future directions. International Journal of Chronic Obstructive Pulmonary disease. 2019; 14: 1127-1138.
- O’Donnell DE, Milne KM, James MD, et al. Dyspnea in COPD: New mechanistic insights and management implications. Advances in Therapy. 2020; 37: 41-60.
- Laviolette L, Laveneziana P. Dyspnoea: a multidimensional and multidisciplinary approach. European Respiratory Journal. 2014; 43: 1750-1762.
- Davis MD, Montpetit AJ. Exhaled breath condensate. An update. Immunology & Allergy Clinics of North America. 2018; 38: 667-678.
- Davis MD, Fowler SJ, Montpetit AJ. Exhaled breath testing – A tool for the clinician and researcher. Paediatric Respiratory Reviews. 2019; 29: 37-41.
- Khoubnasabjafari M, Rahimpour E, Jouyban A. Exhaled breath condensate as an alternative sample for drug monitoring. Bioanalysis. 2018; 10(2): 61-64.
- Kuban P, Foret F. Exhaled breath condensate: Determination of non-volatile compounds and their potential for clinical diagnosis and monitoring. A review. Analytica Chimica Acta. 2013; 805: 1-18.
- Muza M, Konieczna L, Baczek T. A review of the usefulness of non-invasive exhaled breath condensate pH analysis for diseases diagnosis with elements of meta-analysis: An update from 2012. Pharmaceutica Analytica Acta. 2019; 10(1): 605. doi:10.35248/2153-2435.19.10.605
- Konstantinidi EM, Lappas AS, Tzortzi AS, et al. Exhaled breath condensate: Technical and diagnostic aspects. Scientific World Journal. 2015. doi: 10.1155/2015/435160
- Bajaj P, Ishmael FT. Exhaled Breath Condensates as a Source for Biomarkers for Characterization of Inflammatory Lung Diseases. Journal of Analytical Sciences, Methods & Instrumentation. 2013; 3: 17-29.
- Rahimpour E, Khoubnasabjafari M, Jouyban-Gharamaleki V, et al. Non-volatile compounds in exhaled breath condensate: review of methodological aspects. Analytical and Bioanalytical Chemistry. 2018; 410(25): 6411-6440.
- Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. Journal of Allergy & Clinical Immunology. 2016; 138(1): 16-27.
- Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox Biology. 2020; 33; 101544. doi:10.1016/j.redox.2020.101544
- Higham A, Quinn AM, Cançado JED, et al. The pathology of small airways disease in COPD: historical aspects and future directions. Respiratory Research. 2019; 20(1): 49. doi.org/10.1186/s12931-019-1017-y
- Nishino T. Dyspnea: underlying mechanisms and treatment. British Journal of Anaesthesia. 2011; 106(4): 463-474
- Anzueto A, Miravitlles M. Pathophysiology of dyspnea in COPD. Postgraduate Medicine. 2017; 129(3): 366-374.
- Banzett RB, O’Donnell CR. Should we measure dyspnea in everyone? European Respiratory Journal. 2014; 43: 1547-1550.
- Lazar Z, Horvath I, Vestbo J, et al. Exhaled breath condensate in chronic obstructive pulmonary disease: methodological challenges and clinical application. Minerva Pneumologica. 2018; 57(2): 42-56.
- Basu S. F2-isoprostanes in human health and diseases: From molecular mechanisms to clinical implications. Antioxidants & Redox Signaling. 2008; 10(8): 1406-1426.
- Ahmadzai H, Huang S, Hettiarachchi R, et al. Exhaled breath condensate: a comprehensive review. Clinical Chemistry & Laboratory Medicine. 2013; 51(7): 1343-1361.
- Montuschi P. Measurement of biomarkers of oxidative stress and airway inflammation in exhaled breath condensate: Methodology and potential applications in patients with COPD and Healthy smokers. Volatile Biomarkers. 2013; 360-381.
- Dodig S, Cepelak I. (2013). Exhaled breath condensate- from an analytical point of view. Biochemia Medica. 2013; 23(3): 281-295.
- Lim MY, Thomas PS. Biomarkers in Exhaled Breath Condensate and Serum of Chronic Obstructive Pulmonary Disease and Non-Small-Cell Lung Cancer. International Journal of Chronic Diseases. 2013. doi: 10.1155/2013/578613
- Liang Y, Yeligar SM, Brown LA. Exhaled breath condensate: a promising source for biomarkers of lung diseases. ScientificWorldJournal. 2012. doi: 10.1100/2012/217518
- Murata K, Fujimoto K, Kitaguchi Y, et al. Hydrogen peroxide content and pH of exhaled breath condensate for patients with asthma and COPD. Journal of Chronic Obstructive Pulmonary Disease. 2014; 11(1): 81-87.
- Inonu H, Doruk S, Sahin S, et al. Oxidative stress levels in exhaled breath condensate associated with COPD and smoking. Respiratory Care. 2012; 57(3): 413-419.
- Cavalcante AM, de Bruin PC, de Bruin VMS, et al. Melatonin reduces lung oxidative stress in patients with chronic obstructive pulmonary disease: a randomized double-blind, placebo-control study. Journal of Pineal Research. 2012; 53(2): 238-244.
- Chhabra SK, Gupta M. Exhaled breath condensate analysis in chronic obstructive pulmonary disease. Indian Journal of Chest Diseases and Allied Sciences. 2012; 54: 27-37.
- Ashmawi SSA, Dewdar IA, Mohamed NA, et al. Measurement of 8-isoprostane in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Egyptian Journal of Chest Diseases & Tuberculosis. 2018; 67(3): 226-230.
- Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD – implications and relevance for treatment. International Journal of Chronic Obstructive Pulmonary Disease. 2014; 9: 1207-1224.
- Garci-Rio F, Romero D, Lores V, et al. Dynamic hyperinflation, arterial blood oxygen and airway oxidative stress in stable patients with COPD. Chest. 2011; 140(4): 961-969.
- Makris D, Paraskakis E, Korakas P, et al. Exhaled breath condensate 8-isoprostane, clinical parameters, radiological indices and airway inflammation in COPD. Respiration. 2008; 75: 138-144
- Fukushi I, Okada Y. Mechanism of dyspnea sensation: A comprehensive review for better practice and of pulmonary rehabilitation. Journal of Rehabilitation Neuroscience. 2019; 19(1): 22-32.
- Undem BJ, Nassenstein C. Airway nerves and dyspnea associated with inflammatory airway disease. Respiratory Physiology & Neurobiology. 2009; 167: 36-44.
- Taylor-Clarka TE, Undemb BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Respiratory Physiology & Neurobiology. 2011; 178(3): 406-413.
- Horváth I, Barnes PJ, Loukides S, et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. European Respiratory Journal. 2017; 49(4): 1600965. doi: 10.1183/13993003.00965-2016
