Efficacy of methanol extract of Zingiber officinale rhizome against acute pneumonia caused by Pseudomonas aeruginosa
Ankita S Chakotiya1, Alka Narula3, Rakesh K Sharma2*
1Division of CBRN Defence, Institute of Nuclear Medicine and Allied Sciences, Delhi - 110054, India
2Defence Food Research Laboratory, Mysuru – 570011, India
3Department of Biotechnology, Jamia Hamdard, Delhi - 110062, India
Abstract
Pseudomonas aeruginosa has become one of the major threats to public health as it causes serious diseases like pneumonia and the condition becomes sever if the disease is caused by drug-resistant strain. Thus, in the present study, the antibacterial activity of Zingiber officinale against P. aeruginosa causing a lung infection in Swiss albino mice was evaluated. Disease analysis includes bacteremia, x-rays based observation of patches formation in lungs, and destruction of lung tissue. Bacterial load showed a significant decline in the herbal treated group up to 1.13 ± 0.2 Log10 CFU/ml at the 7th day as compared to the untreated group with the bacterial burden 3.0 ± 0.12 Log10 CFU/ml. The histopathology reveals more distorted alveoli in an infected group with an accumulation of immune cells while in the treated group improved lung histology was seen. The findings stated that Z. officinale is effective may provide a suitable lead for future development and possible clinical utility as inhibitors of lung infection caused by P. aeruginosa.
Introduction
Nosocomial pneumonia is a serious ailment in patients with more than 48 h of stay in hospital1. The nosocomial pneumonia is the second most common nosocomial infection associated with intensive care unit (ICU)2. The most common cause of nosocomial pneumonia is microaspiration of etiological agents that includes predominantly (50–80%) gram-negative bacteria such as P. aeruginosa (17.4%), K. pneumonia, Enterobacter spp. (18.1%), and Haemophilus influenzae (4.9%) and gram-positive bacteria such as Staphylococcus aureus (18%)2. P. aeruginosa is a frequently isolated pathogen from the clinical sample of hospitalized patients thus is responsible for a high percentage of infections3. This bacterium shows antibiotic resistance, by developing membrane impermeability, efflux pump systems and changes in drug targets thus posing a huge remedial problem4-6. WHO on 28 February 2017 have declared this bacterium as a critical antibiotic-resistant pathogen that requires the urgent need of a new antibiotic. All such devastating scenarios has confirmed that P. aeruginosa is definitely one of the critical pathogens with unmanageable virulence determinants. Additionally, this pathogen is also re-emerging with exhibiting trends of extensive drug resistance, which might lead to pan-drug resistance scenarios in the coming future, if not managed at present.
The exploration of the utility of ethnopharmacological importance of medicinal plants is always a research quest to resolve this challenging issue of multi-drug resistance. Natural plant product(s) in the form of the pure compound or as the standardized extracts may provide an alternative to develop potent leads.
Zingiber officinale (ginger) is a perennial tropical herbaceous rhizome and a native to Asia7. China is known to be the first for the cultivation of Z. officinale then spread to India, South East Asia, West Africa and the Caribbean7. But in terms of its production, India is the biggest producer of Z. officinale in the world. In India, it is cultivated in almost all the states majorly in Orissa, West Bengal, North eastern states, and Kerala owing to favorable climatic condition7. Since ancient times the ginger is in use as a medicinal plant and used in Ayurvedic and Chinese medicine for curing heart problems, treat stomach disorders, diarrhoea, and nausea7. It is also reported to be enriched with anti-oxidant, immunomodulatory, anti-inflammatory, anti-rheumatic, anti-hepatotoxic, etc. medicinal traits7. The antimicrobial activity of ginger against gram positive, gram negative bacteria and fungi have been reported. The crude extract of Z. officinale was found to be lethal against P. aeruginosa as it causes membrane disruption and supressees the efflux activity and biofilm formation at in vitro level8. In order to build up novel therapeutic modalities for the lung infection caused by P. aeruginosa, the effect of crude extract of herbal on the infection was evaluated in this study.
Materials and Methods
Isolation and identification of test organism
The isolation of P. aeruginosa from a medical sample was done in the Department of Microbiology, National Centre for Disease Control (NCDC), New Delhi, India. The battery of tests was performed for the recognition of P. aeruginosa from the sample as described in the previous studies9. The isolated bacterium was inoculated in sterile nutrient broth (at 37°C for 48 h). The antibiotic susceptibility test was performed by using Kirby Bauer disc diffusion technique in our previous studies, to determine the drug susceptibility pattern9. The result of the test was interpreted according to the Clinical Laboratory Standard Institute guidelines (CLSI) in 2008.
Inoculum preparation
The fresh sterile nutrient broth was inoculated with the loop full of culture of P. aeruginosa grown on Mueller Hinton agar plate and incubated at 37°C for 17-18 h. 0.5 McFarland standard was used to maintain the turbidity by standard procedure. This suspension was used as inoculum.
Preparation of herbal extract
250 g of shade-dried plant materials (rhizome) were procured from a reputed supplier of medicinal herbs in New Delhi, India. Hot continuous percolation method at 65°C temperature was used to prepare aqua-alcoholic (water: methanol; 30:70) rhizome extract of Z. officinale9.
Liquid Chromatography Mass Spectrometry Analysis
LC-MS analysis was performed using a LCQ MS mass spectrometer (Thermo-Finnigan, India). The solution of crude extract in methanol was analyzed on Acquity TQD QBB 1152 using Thermo Scientific™ Accucore™ RP-MS analytical column (100 x 2.1 mm, 2.6 µm) with auto-injector (SIL-10 AD VP) and diode array detector (SPD M-10 A). 0.1% formic acid in water as mobile phase was used at a flow rate of 400 µL/min for the elution. 30 and 650 L/h flow rate were set for the cone gas and desolvation, respectively. The desolvation temperature was 350°C. In the range of 150-1500 Dalton (Da), the positive and negative ions were scanned. The mass spectral data of reference compound(s) was used to identify known compounds in the crude extract by comparative analysis of the obtained peaks.
Animals
The mammalian model system selected for investigation of the antibacterial efficacy of the herbal extract (Z. officinale) was Swiss albino Strain’A’ mice. The experiments were conducted as per the guideline laid down by the Institutional Animal Ethics Committee (IAEC) of INMAS (Reg. No. 8/GO/a/99/CPCSEA)10. Swiss albino Strain ‘A’ male mice (06-07 weeks) weighing about 30 ± 2 g were obtained from INMAS animal facility and maintained under the controlled laboratory conditions. The animals were housed in fresh air conditioned room with temperature 25°C and the relative humidity 60 ± 5% RH. The animals were fed standard animal food pellets (Amrut Laboratory Animal Feed, India) and were offered drinking water. For the administration of herbal extract dissolved in drinking water, the oral route was chosen.
Standardization of lung infection caused by P. aeruginosa in mice model
The empirical dose estimate for causing such infections using microbial count is ~107 CFU/mL11. Evidently, 107 CFU/mL was lethal dose while the expected outcome designed for the study was a non-lethal lung infection. Optimization and classical literature surge revealed that 105 CFU/mL could provide such outcome, accordingly chosen for the study,sup>12
.
Infection Protocol: Mice were administered anesthesia (xylocaine) subcutaneously and infected intratracheally. Intratracheal instillation was performed with 0.1 mL inoculums (1 × 105 CFU/mL) suspension via 29 gauge needle on an insulin syringe. Pathogenesis was characterized by studying different parameters including (a) temperature, (b) body weight, (c) nasal secretion, (d) piloerection, (e) locomotion, (f) grooming and (g) body condition were assessed. Blood was collected at the time interval (2nd, 4th, 8th, 24th, 28th, and 32nd h).
Acute toxicity by Maximum Tolerable Dose (MTD) Assay
Limit test: The experimentation was done in order to evaluate the acute toxicity of the extract in terms of percent survival, and gross changes in behavior alterations. A study was performed over a period of 24 h following the oral administration of single dose of 2000 mg/kg body weight with six animals (male mice). The mice were constantly observed for the first four hours in order to visualize signs of toxicity and behavioral changes. The maximum dose up to which no lethality was observed referred as Maximum Tolerable Dose (MTD)13.
In vivo antipseudomonal activity
Animals were divided randomly into 03 groups (control or no infection, negative control or untreated and D1 i.e., herbal treated) each group contains 06 mice. 2% Xylocaine was used to anaesthise the mice by giving local anesthesia subcutaneously, and the intratracheal route was used to administer infection via a 29- gauge needle. 100 µl of a suspension of bacterial cells (105 CFU/mL) in early-logarithmic-phase12. The herbal treatment was given twice a day at the interval of 2 and 4 h of post-inoculation on the same day. The treatment included an oral dose of D1= 98 mg/kg body weight and continued for 7 days. Bacteremia was evaluated in terms of CFU/mL for this, blood sample was collected from untreated and herbal treated group once daily and streaked on agar plate. Radiological imaging of lungs was done prior to killing at day 7. The lungs were aseptically collected and were analyzed for the histopathological study. The biomedical waste bags were used to pack the dead animals after experiment further these bags were picked up by Delhi Pollution Control Committee authorized Biomedical waste disposal vehicle for the incineration of the dead body of animals at their own facility.
Radiological study
The radiology imaging of lungs of mice was performed. Subcutaneously ketamine was given and the anaesthetized mice were placed on a radio-graphic sensor (Krystal X easy digital, Owandy software) at a distance of 80 cm from the X-ray source. Radiographic assessment was done by using X-ray machine (Intra Scan DC Skan Ray, with IV- 1 phase 230 V, IF- 50 Hz, Momentary & Stand by current -0.25A, Made in India) with a 48 kVp exposure for 0.5 mAs.
Hematology
Three animals from each group were used to conduct hematological and biochemical testing. Samples of blood (200 µL) for hematological and biochemical analysis was withdrawn from saphenous vein in a 3 mL ethylene-diamino-tetracetic-acid (K3-EDTA) tube (Becton Dickinson, BD Vacutainer). Blood sample was then analyzed for complete blood profile including red blood cell (RBC) count, hemoglobin, platelet count, total and differential white blood cell (WBC) count. The measurements were executed by Hematology Analyzer (Medonic CA530).
Histopathology
The cervical dislocation was used to sacrifice the mice on 7th day of experiment. The whole lungs were expunged and fixed in 4% paraformaldehyde followed by decalcification in 5% formic acid. The lungs were embedded in paraffin for tissue sectioning. Haematoxylin–eosin stains were used for staining the tissue sections (5 µm thick) and these sections were observed under Olympus microscope (Olympus U-TV 0.63×C; SN 9L01588 T2, Tokyo, Japan (Made in the Philippines).
Statistical analysis
Results are expressed as Mean ± SEM. Statistical analysis of the data was done by applying the analysis of variance (ANOVA), followed by Turkey’s test for all parameters. The p < 0.05 was considered significant.
Results
Liquid Chromatography Mass Spectrometry Analysis
Mass spectral analysis of the eluted samples was performed in order to record fragmentation pattern. LCMS analysis revealed 09 peaks out of which 04 peaks were recorded and the identified compounds were 8-gingerol (RT: 4.12), 6-gingerol (RT: 5.25), paradol (RT: 6.12) and 6-shogaol (RT: 8.48) (Fig.1). In addition to this 05 unidentified peaks were also obtained.
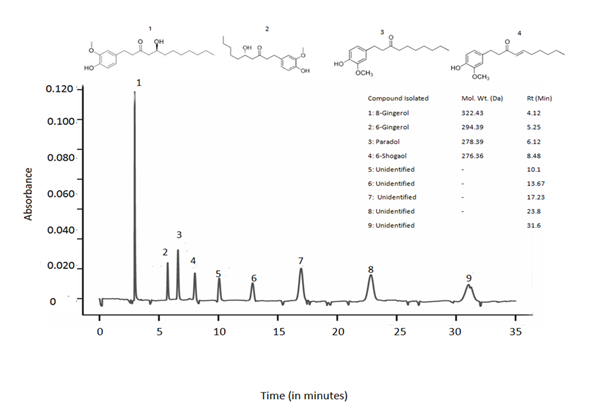
Fig 1. LCMS profile of crude extract of Zingiber officinale
HPLC chromatogram of the major phytocompounds of extract of Zingiber officinale detected at 278 nm.
Standardization of lung infection caused by P.aeruginosa in mice model
A significant bacterial load of 3.53 ± 0.17 Log10 CFU/ml was recorded at 48th h of post-infection (Fig 2). A weight loss up to 32% was observed at 48th h with significant Hypothermia with the temperature drop of 27 ± 0.5 °C (48th h) from 38.47 ± 0.69°C (0 h) (Fig 3). Absence of grooming was observed, eyes were partially opened, very less locomotion and weakened after 24 h. From 8th h nasal discharge along with piloerection stays, Hyperventilation after 4th h of inducing infection was noticed upto one day of infection cycle and later it goes down.
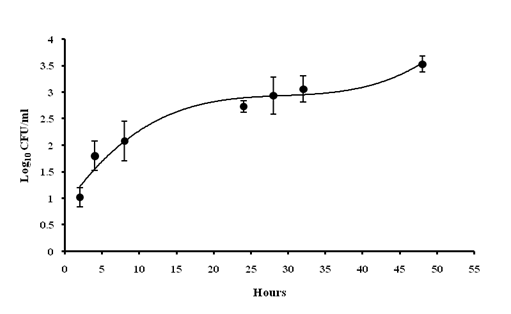
Fig 2. Assessment of bacterial load of blood: The number of bacterial colonies was found to be increases gradually just after 2 hr of post-inoculation
Values are expressed as mean ± standard deviation of experiments each performed in triplicates and analysed using univariate ANOVA.
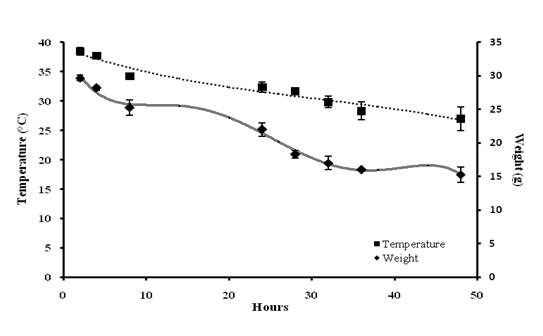
Fig 3. Evaluation of physical parameters: Decrease in body temperature commenced at 4th h of post-inoculation; Persistent decrease in weight was also observed
Values are expressed as mean ± standard deviation of experiments each performed in triplicates and analysed using univariate ANOVA.
Maximum Tolerable Dose assay
In order to perform the preclinical animal studies the lethal dose or maximum tolerated dose of the extract was determined. As shown in the Table 1, no mortality was observed in the mice at 2000 mg/kg body weight, administered orally. Also, no significant changes were observed in the physical parameter and no any adverse effects were observed on mice.
In vivo antimicrobial activity
After 2h of the commencement of herbal treatment the bacterial burden was recorded 1.02 ± 0.27 and 1.04 ± 0.17 Log10 CFU/ml for negative control (infected), and D1 group, respectively. A gradual increase of bacterial load in the infected group was observed that reaches up to 3.0 ± 0.12 Log10 CFU/ml. A significant decline of the bacterial loads in blood and reduces by 1.13 ± 0.2 Log10 CFU/ml at 7th day (Fig. 4). It was observed that all the animals have recovered from the infection with herbal dose D1. In negative control group all the animals died on day 7 of the study.
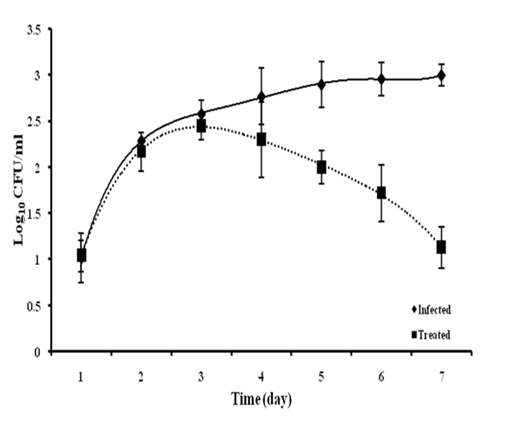
Fig 4. Radiological analysis of thoracic cage
(A) Lesions were observed in infected group (under circle), (B) Normal X-ray image is obtained with uninfected group, (C) Mild lesions were obtained
Radiological study
A severe soft tissue damage of lungs was observed in radiological analysis of the negative control (untreated) group (Fig. 5A). The herbal was found to be capable of seizing and healing the tissue damage (Fig. 5C) as moderate tissue damage was observed in the form of lesions in treated group. In case of uninfected group normal image of lungs was observed (Fig. 5B).
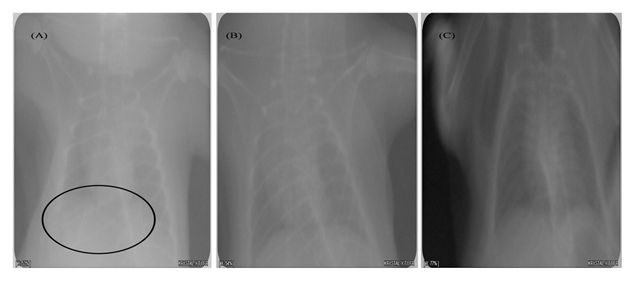
Fig 5. In vivo antibacterial activity of Zingiber officinale extract
Bacteremia in two groups (Infected and Treated) of mice, observed at pre-decided time interval for the study
Hematology
There were changes observed in the hematological parameters during the dosing period. An increased level of white blood cells (12.65 ± 0.12 × 103/µL) was observed in case of infected group during the study while in treated groups no such significant changes were observed. WBC count peaked in the infected group with significant raise in lymphocytes as compared to not infected group as well as treated group. Neutrophils percentage was also peaked in infected group with the value 78.89 ± 1.8 % as compared to other groups in the study. There is are no aberrations were observed in count of RBC, eosinophils, and monocytes and other hematological parameters like hemoglobin in all the groups (Table 2).
Histopathology
Histopathology of lungs showed more diffuse and patchy accumulation of inflammatory cells within the alveolar space along with the infiltrates was noted in all the lung section of infected mice (Fig. 6A). No exudate was observed in uninfected mice also the alveoli were found to be intact with undamaged lung parenchyma (Fig. 6B). In herbal treated animal group improved lung histology was observed with the disappearing of the exudates from the lungs. Also, the inflammatory cell infiltrates of bronchi and perivascular edema were less seen in the examined section of herbal treated group (Fig. 6C).
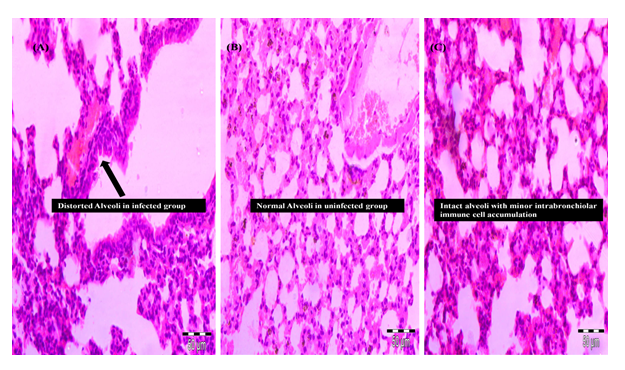
Fig 6. Lung pathohistology photomicrographs
(A) Severe histological damage occurs in lungs in particular massive inflammatory infiltrates within the alveolae and alveolar damage (arrow), (B) Normal lung histology in uninfected mice with intact alveoli (arrow), (C) Intact alveoli with normal lung histology in treated (D1) group with minor intrabronchiolar immune cell accumulation
Discussion
Antibiotics have been established as the mainstay in the treatment of bacterial infections, there was a conviction in the medical field that this would gradually eradicate the onset of infectious diseases. However, the world wide emergence of such bacterial strain has become a customary problem. WHO in February 2017 released a list of antibiotic resistant bacteria and categorized them into critical, high and moderate severity agents posing a threat to human health. P. aeruginosa has been placed in the critical category as it has been reported to account for higher morbidity and mortality rate in humans. This bacterium is an opportunistic pathogen that accounts for a high percentage of nosocomial pneumonia. No absolute treatment modality is available for MDR P. aeruginosa. Additionally, all the available 3rd line drugs exhibit serious hepato-renal toxicities. Numerous natural plant products have also been tested for their antibacterial efficacy against P. aerugionsa across the globe.
We have reported previously 05 herbals against multi-drug resistant P. aeruginosa by utilizing bioprospection approach followed by validation by using molecular docking analysis8. The aquo-alcoholic extract of rhizome of Z. officinale was also assessed at in vitro level to determine its Minimum Inhibitory Concentration (10µg/mL) and their mechanism of activity were additionally assessed to repress the biofilm formation and efflux movement of the pathogen9. The present study concentrates on the assessment of various parameters of disease and affirmation of efficacy of the herbal extract by utilizing a pre-clinical pneumonic murine model.
MTD was determined to estimate the highest dose of the herbal that does not cause unacceptable side effects. Limit test was performed in order to determine the MTD by using OECD guideline 423, wherein, the results revealed that the aquo-methanolic extract of Z. officinale have not been found to be toxic with an oral dose of 2000 mg/kg body weight with no significant morbidities. No aberrations were observed in the body weight and other physical parameters (Table 1). Lethargy and diarrhea did not occur in animals. Thus, it is concluded that the extracts does not show any toxic effects.
Table 1. Acute toxicity study
| Herbal | No. of animal (male mice) | Dose (mg/kg body weight oral) | Observations | |||||
|---|---|---|---|---|---|---|---|---|
| Mortality | Body weight | Temperature | Behavioral pattern | Lethargy | Diarrhea | |||
| Z. officinale | 06 | 2000 | Not observed | No significant changes | No significant changes | Active | Not observed | Not observed |
To study the effect of the herbal extract on a pulmonary infection model, first of all the pathogenesis of P. aeruginosa was determined. The animal was inoculated through intratracheal instillation in order to bypass the upper airways and to cause acute pneumonia thereby delivering a high load of bacteria in the distal bronchi. Similar route was used by Munder and coworkers, to study the acute P. aeruginosa lung infection14. The virulence of the pathogen is measured by pathogenesis in mice and hypothermia along with bacteremia which was studied as an accurate predictor of illness. Soothil et al., (1992) also studied the hypothermia for the measurement of bacterial virulence11. The pathological manifestation was observed from 2nd h of post-inoculation by observing the bacteremia, hypothermia and weight loss (Fig 2 and 3). As it is mentioned in the literature that the bacteremia often arises in pneumonia and is associated with the high rate of morbidity and mortality15. The occurrence of bacteremia was employed as the primary end point. A sudden drop in body temperature and significant decline in weight were found in all the infected animals. These findings are in line with the symptoms obtained by Soothil et al., in their study on mice11. Exposure to P. aeruginosa did not cause fever, but it leads to hypothermia, as reported by Munder and coworkers14.
At day 7 of post-administration of herbal extract there is a significant drop of bacterial count of P. aeruginosa was observed. X-rays analysis was used for the assessment of the progression of pneumonia in mice (Fig 5). The patchy parenchymal tissue in the x-rays report of the lungs suggesting the presence of pneumonia (Fig. 5A). At the termination point of the study the lungs were removed from euthanized mice shows and there have been numerous distinct areas of hemorrhage and alveolar inflammation was observed.
There were significant changes observed in the hematological parameters during the dosing period. An increased level of white blood cells (12.65 ± 0.12 × 103/µL) was observed in case of infected group during the study while in treated groups no such significant changes were observed. WBC count peaked in the infected group with the significant rise in lymphocytes as compared to the control group as well as the treated group. It has been reported that high WBC count is associated with pneumonia as well as Chronic Obstructive Pulmonary Disease (COPD). Gan et al., pointed out that there is a direct relationship between the high WBC count and induction of pulmonary disease that demonstrated the severity of exacerbation16. Sin et al., reported the association between high blood WBC count with an increased severity of symptoms in case of pulmonary diseases17. Thus, it is reasonable to check WBC count as it presents a physiological marker for lower respiratory disease18. The neutrophil percentage was also found to be increasing in the infected group with the value 78.89 ± 1.8 % as compared to other groups in the study. Neutrophil accumulation during lower respiratory tract bacterial infection was also demonstrated by Mizgerd (2002) in mice as one of the innate immune response in the lung against bacterial infection19. There is no aberrations were observed in count of RBC, eosinophils, monocytes and hemoglobin in all the groups (Table 2).
Table 2. Hematological profile
| Parameters | No infection | Only infection | Infection + Z. officinale dose | |
|---|---|---|---|---|
| Haemoglobin (g percentage) | 14. 13 ± 1.68 | 14.98 ± 1.67 | 14.23 ± 1.78 | |
| RBC count (×106/µl) | 5.75 ± 1.82 | 5.55 ± 1.79 | 5.35 ± 1.52 | |
| Platelet count (×106/µl) | 8.47 ± 2.88 | 8.31 ± 1.08 | 8.7 ± 1.80 | |
| Total WBC (×103/µl) | 5.37 ± 0.84 | 12.65 ± 0.12 | 6.37 ± 0.84 | |
| Differential percentage | Neutrophil | 20.67 ± 0.80 | 78.89 ± 1.8 | 20.37 ± 0.59 |
| Lymphocytes | 88.2 ± 1.84 | 121 ± 2.4 | 87. 89 ± 2.34 | |
| Eosinophil | 2.17 ± 0.75 | 2.07 ± 0.15 | 2.7 ± 0.75 | |
| Monocyte | 0.67 ± 0.28 | 0.65 ± 0.38 | 0.65 ± 0.08 |
Severe damage with infiltration of exudates in the alveoli was observed during histopathological examination (Fig. 4A). In herbal treated group there were better condition of lung tissues was obtained with normal to mild inflammation as compared to untreated group (Fig. 4C). The anti-inflammatory action during pneumonia along with the bactericidal activity of Z. officinale was observed by the reduced tissue inflammation in the treated group. Thus, the findings indicating the protective effect and symptomatic relief providing capabilities of the Z. officinale extract.
In any case, no preclinical efficacy investigations of Z. officinale focusing on P. aeruginosa or its drug resistant strains causing pneumonia have yet been conducted.
LCMS analysis of the rhizome extract had confirmed the presence of alkaloids mainly (Fig 1). Gingerol have been known to possess antibacterial activity against periodontal bacteria and vancomycin-resistant enterococci20-21. Also phenolic compounds of ginger have been reported to show the therapeutic potency against cancer and show activity in cell signaling pathway22-23.
Acknowledgement
The authors are grateful to Director, INMAS; Director, National Centre for Disease Control, Delhi for provision of research facilities and active support. The present study conducted under the INMAS sub-programme [TD-15/DRD-203 (INM-318)] of ‘Samarthya’ Programme of DRDO is duly acknowledged. ASC is grateful to UGC for the award of fellowhip(2-13/2002).
Funding: The work is supported by University Grant Commission (2-13/2002).
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: The experiments were conducted as per the guideline laid down by the Institutional Animal Ethics Committee (IAEC) of INMAS (Reg. No. 8/GO/a/99/CPCSEA)
References
- Yang YW, Jiang YZ, Hsu CM, et al. Pseudomonas aeruginosa Ventilator-Associated Pneumonia Induces Lung Injury through TNF-α/c-Jun NH2-Terminal Kinase Pathways. PLoSONE. 2016; 12: 1.
- Rotstein C, Evans G, Born A, et al. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can J Infect Dis Med Microbiol. 2008; 19: 20.
- Jemima Kingsley SA, Verghese S. First Report of OXA-4, an ESBL Isolated from Pseudomonas aeruginosa a South Indian Strain. Indian J Med Microbiol. 2013; 53: 308.
- Jacqueline A, Roquilly C, Desessard D, et al. Efficacy of ceftolozane in a murine model of Pseudomonas aeruginosa acute pneumonia: in vivo antimicrobial activity and impact on host inflammatory response. J Antimicrob Chemother. 2013; 68: 177.
- Livermore DM. Of Pseudomonas aeruginosa porins, pumps and carbapenems. J Antimicrob Chemother. 2001; 47: 247.
- Garvey MI, Rahman MM, Gibbon S, et al. Medicinal plant extracts with efflux inhibitory activity against Gram-negative bacteria. Int J Antimicrob Agents. 2011; 37: 145.
- Shukla Y, Singh M. Cancer preventive properties of zinger: a brief review. Food Chem Toxicol. 2007; 45: 683.
- Chakotiya AS, Tanwar A, Narula A, et al. Zingiber officinale: Its antibacterial activity on Pseudomonas aeruginosa and mode of action evaluated by flow cytometry. Microb Path. 2017; 107: 254.
- Chakotiya AS, Chawla R, Thakur P, et al. In vitro activity of promising nutraceuticals for targeting multi-drug resistant Pseudomonas aeruginosa. Nutrition. 2016; 32: 890.
- Badyal DK, Desai C. Animal use in pharmacology education and research: the changing scenario. Indian J Pharmacol, 46 (2014) 257.
- Soothil JS, Morton DB, Ahmad A. The HID 50 (hypothermia-inducing dose 50): an alternative to LD50 for the measurement of bacterial virulence. Int J Exp Path. 1992; 73: 95.
- Chakotiya AS, Tanwar A, Narula A, et al. Effect of aquo-alchoholic extract of Glycyrrhiza glabra against Pseudomonas aeruginosa in Mice Lung Infection Model. Biomed Pharmacother. 2017; 90: 171.
- Mir MA, Sawhney SS, Jassal MMS. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker J Pharm Pharmacol. 2013; 2: 1.
- Munder A, Wölbeling F, Kerber-Momot T, et al. Acute intratracheal Pseudomonas aeruginosa infection in cystic fibrosis mice is age-independent. Resp Res. 2011; 12: 1.
- Reines HD, Cook FV. Pneumonia and bacteremia due to Aeromonas hydrophila. Chest. 1981; 80: 264.
- Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax. 2004; 59: 574.
- Sin Don D, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases. Circulation. 2000; 107: 1511.
- Tatar D, Gunes S, Anar C, et al. Markers of lower respiratory tract infections in emergency departments. Multidiscip. Respir Med. 2013; 8: 1.
- Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol. 2002; 14: 123.
- Park M, Bae J, Lee DS. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytother Res. 2008; 22: 1446.
- Nagoshi C, Shiota S, Kuroda T, et al. Synergistic effect of [10]-gingerol and aminoglycosides against vancomycin-resistant enterococci (VRE). Biol Pharm Bull. 2006; 29: 443.
- Schadich E, Hlavá? J, Volná T, et al. Effects of Ginger Phenylpropanoids and Quercetin on Nrf2-ARE Pathway in Human BJ Fibroblasts and HaCaT Keratinocytes. Biomed Res Int. 2016; 2016: 2173275.
- Kundu JK, Na HK, Surh Y. Ginger-derived phenolic substances with cancer preventive and therapeutic potential. Forum of Nutrition. 2009; (61): 182.
